Enlivex CEO Issues Letter to Shareholders Outlining Strategic Roadmap Following Positive Phase IIa Allocetra™ Results
| Allocetra™ demonstrated statistically significant and clinically meaningful improvements in pain and function in Phase IIa trial in primary osteoarthritis patients | |
| Six-month data expected November 2025; Phase IIb trial initiation planned for Q2 2026 |
Nes-Ziona, Israel, Sept. 11, 2025 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV, the “Enlivex”), a clinical-stage macrophage reprogramming immunotherapy company, today issued the following update to shareholders from Chief Executive Officer, Oren Hershkovitz, highlighting the strength of its recently announced Phase IIa topline results for Allocetra™ in knee osteoarthritis (KOA) and detailing the next steps planned for its clinical development roadmap.
Dear fellow shareholders,
I would like to take this opportunity to provide you with our perspective on our recent Phase IIa (ENX-CL-05-001) 3-month topline data readout for Allocetra™ in patients with moderate-to-severe KOA.
We believe that these three-month results represent a key positive milestone for Enlivex. After a careful analysis of the data and discussions with multiple physicians and experts who are among the world-leaders in the area of KOA, we believe that Allocetra™ has the potential to become a leading therapy of choice for the tens of millions of patients with primary (idiopathic, age-related) KOA, who currently have few and poor treatment options for this debilitating disease.
Having received many calls from investors after announcing the positive 3-month data, we would like to reiterate that we believe that, notwithstanding recent stock price movements, the clinical data and expert validation are clear. We further believe Allocetra™ is well positioned to advance toward late-stage development and ultimately address a major unmet need in KOA.
Positive Feedback from Analysts and Key Opinion Leaders Reinforce Confidence in Allocetra™
To underscore this, it is important to highlight the enthusiasm not only from our team but also from respected industry analysts and key opinion leaders. One of those analysts is Jason Kolbert, M.Sc., a long-time biotech analyst who covers Enlivex. He started his August 22, 2025, report by stating, “Enlivex reported what we, along with every expert we consulted, viewed not just as good but truly exceptional data in its knee osteoarthritis program”. He continues to provide insight into what has technically transpired, and I highly recommend that you obtain a full copy of his research report, as well as the recent H.C. Wainwright research report from Raghuram Selvaraju, Ph.D., a notable veteran life sciences analyst, dated September 2, 2025 discussing the positive results.
Highly positive and encouraging feedback came up during our discussions of the 3-month results with key opinion leaders in KOA. Prof. Ali Mobasheri, a Professor of Musculoskeletal Biology in the Faculty of Medicine, University of Oulu, Finland, is one of world’s leading experts on osteoarthritis and has been serving as a clinical advisor to Enlivex for the last two years. He is the former President of the Osteoarthritis Research Society International (OARSI), the leading medical society for advancing the understanding, early detection, treatment and prevention of osteoarthritis. Prof. Mobasheri participated in, and consulted on, most clinical trials in osteoarthritis during the last 15 years. When we asked for Prof. Mobasheri’s insight on the trial’s 3-month data, he stated, “this positive effect that you have seen in the older patient population is quite intriguing, and it fits nicely with the distinct type of age-related osteoarthritis that is highly prevalent in that population. It is very impressive to obtain such statistical significance in a relatively small patient population, and I highly recommend that you move forward into the next Phase IIb. You may have something here that could change the life of OA idiopathic patients, and I urge you to continue to explore this.”
Key Study Findings Highlight Allocetra™’s Potential in Patients with Age-Related Osteoarthritis
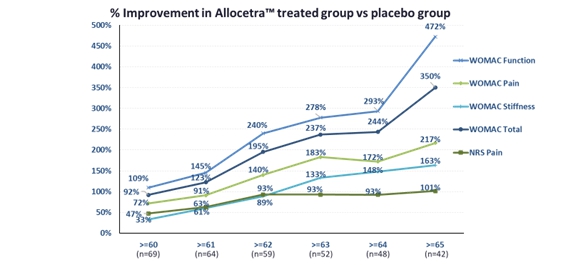
I would like to reiterate some of the key 3-month findings of the study. In addition to assessing the overall safety and efficacy of Allocetra, the study included assessments aiming to find a high-responder patient population, because the term “osteoarthritis” represents a collection of clinical conditions rather than one molecular disease. This means there are multiple pathways for inflammation in osteoarthritis, and AllocetraTM may be suitable for specific pathways, taking into consideration its proposed mechanism of action. This objective was met, and in primary idiopathic age-related osteoarthritis patients (≥60 years), a population representing more than half of the total KOA market and 54% of the study population, Allocetra™ achieved substantial, clinically meaningful and highly statistically significant improvement compared to the control placebo group in pain and function. Efficacy was assessed by the FDA-accepted questionnaire assessing knee pain, function and stiffness (WOMAC -Western Ontario and McMaster Universities Arthritis Index, a validated patient reported questionnaire assessing pain, function and stiffness).
The treatment effect as represented in the reduction of pain and improvement in function comprises potential Phase III primary endpoints are commonly accepted by the FDA. The following results were observed:
- 72% reduction in pain vs. placebo and absolute reduction of 49% from baseline in the AllocetraTM arm
- 109% improvement in function vs. placebo and absolute reduction of 50% from baseline in the AllocetraTM arm
- Efficacy exceeding FDA thresholds for Phase III trials
These improvements demonstrated even greater effects and statistical significance with aging, as we further enrich the responder primary idiopathic age-related osteoarthritis patients.
Allocetra™ was well tolerated, with no drug-related serious adverse events, and mostly mild-to-moderate, transient local reactions were observed.
Significance for the Knee Osteoarthritis Market
KOA is one of the most prevalent and disabling diseases globally, affecting more than 32 million Americans today and projected to impact 78 million by 2040. Despite this enormous burden, there are no approved disease-modifying treatments, with current options limited to pain relief, steroids, or surgery. The prevalence of KOA increases with age, as the progressive degeneration of the joint surface evolves. By the age of 60 years, the prevalence increases to 30% of the entire population, and 50% of patients with knee OA are aged 60 and above. The prevalence of KOA is expected to grow significantly due to the rising geriatric population worldwide and the associated increase in the prevalence of osteoarthritis. This strengthens the importance of the clinical effect observed by AllocetraTM in the age-related primary idiopathic aging population, as a promising therapy that may change the life of a substantial OA population, which is expected to increase over time.
Next Steps and Key Milestones
Building on these strong results, Enlivex is advancing Allocetra™ toward late-stage development with a planned roadmap of catalysts:
-
November 2025: Six-month readout from the ongoing Phase IIa trial
-
2026 (Q1–Q2): Expected regulatory approval of Phase IIb protocol
-
2026 (Q2–Q3): Expected dosing of first patient in Phase IIb trial focused on primary KOA patients
- 2027 (Q2–Q3): Expected three- and six-month topline data from Phase IIb trial
We believe that the positive 3-month topline data from the Phase IIa trial position Enlivex as a strong candidate for a potential partnership with a larger company that has an interest in osteoarthritis, as well as for securing other non-dilutive funding opportunities. We are currently pursuing both.
We remain focused on rapidly executing our clinical roadmap and look forward to potentially unlocking the significant medical and market opportunity, while delivering enduring value for patients and shareholders.
About ENX-CL-05-001
ENX-CL-05-001 is a multi-center Phase I/II clinical trial consisting of two stages. The first stage was a Phase I safety run-in, open-label dose escalation phase to characterize the safety and tolerability of Allocetra™ injections to the target knee, in order to identify the dose and injection regimen for the subsequent Phase IIa stage. The Phase IIa stage is a double-blind, randomized, placebo-controlled multi-centered trial. In addition to evaluating safety, the study protocol was designed to efficiently find a strong signal in a responder population to guide future development, and includes an interim statistical evaluation, conducted by an independent third party and blinded to Enlivex, to assess the potential value of enrollment of up to 50 patients in addition to the original randomized sample size of 130, and its marginal impact on the p-value of the statistical estimation of the total group and specifically to identify a potential responder sub-group. The trial’s key efficacy endpoints evaluate joint-pain and joint-function in comparison to placebo at three months, six months and 12 months post treatment.
ABOUT KNEE OSTEOARTHRITIS
Osteoarthritis is by far the most common form of arthritis, affecting more than 32.5 million Americans and more than 300 million individuals worldwide. About half of knees with ACL injuries develop osteoarthritis within 5 to 15 years. 78 million Americans are projected to have osteoarthritis by the year 2040. Symptomatic knee osteoarthritis is particularly prevalent and disabling, with 40% of men and 47% of women developing knee osteoarthritis in their lifetimes. Osteoarthritis accounts for over one million hospitalizations annually in the United States, primarily for total joint replacement. Osteoarthritis is an heterogenous complex condition and not a single molecular derived disease. This heterogeneity stems from diverse underlying pathologies, such as inflammatory, metabolic, and genetic factors. Recognizing these variations is crucial for developing more personalized oriented treatment rather than a "one-size-fits-all" approach. The burden of osteoarthritis is enormous, and the need for treatments that reduce pain and attendant disability for persons with osteoarthritis is critical. There are currently no medications approved by either the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) that have been demonstrated to arrest, slow or reverse progression of structural damage in the joint.
ABOUT ENLIVEX
Enlivex is a clinical stage macrophage reprogramming immunotherapy company developing Allocetra™, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing and resolution of life-threatening and life debilitating conditions. For more information, visit https://enlivex.com/.
Safe Harbor Statement: This press release contains forward-looking statements, which may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “would,” “could,” “intends,” “estimates,” “suggests,” “target,” “has the potential to” and other words of similar meaning, including statements regarding expected cash balances, expected clinical trial results, market opportunities for the results of current clinical studies and preclinical experiments, the effectiveness of, and market opportunities for, ALLOCETRATM programs. All such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that forward-looking statements involve risks and uncertainties that may affect Enlivex’s business and prospects, including the risks that Enlivex may not succeed in generating any revenues or developing any commercial products; that the products in development may fail, may not achieve the expected results or effectiveness and/or may not generate data that would support the approval or marketing of these products for the indications being studied or for other indications; that ongoing studies may not continue to show substantial or any activity; and other risks and uncertainties that may cause results to differ materially from those set forth in the forward-looking statements. The results of clinical trials in humans may produce results that differ significantly from the results of clinical and other trials in animals. The results of early-stage trials may differ significantly from the results of more developed, later-stage trials. The development of any products using the ALLOCETRATM product line could also be affected by a number of other factors, including unexpected safety, efficacy or manufacturing issues, additional time requirements for data analyses and decision making, the impact of pharmaceutical industry regulation, the impact of competitive products and pricing and the impact of patents and other proprietary rights held by competitors and other third parties. In addition to the risk factors described above, investors should consider the economic, competitive, governmental, technological and other factors discussed in Enlivex’s filings with the Securities and Exchange Commission, including in the Enlivex's most recent Annual Report on Form 20-F filed with the Securities and Exchange Commission. The forward-looking statements contained in this press release speak only as of the date the statements were made, and we do not undertake any obligation to update forward-looking statements, except as required under applicable law.
ENLIVEX CONTACT
Shachar Shlosberger, CFO
Enlivex Therapeutics, Ltd.
shachar@enlivexpharm.com
INVESTOR RELATIONS CONTACT
Dave Gentry, CEO
RedChip Companies Inc.
1-407-644-4256
ENLV@redchip.com

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.